What Are Two Examples Of Unstable Atoms That Can Be Used For Nuclear Fission
Nuclear fission
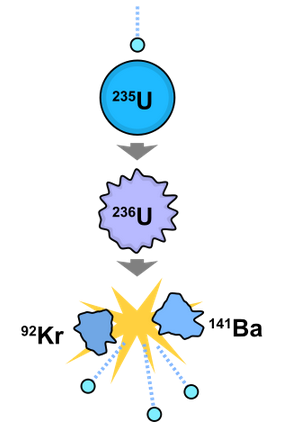
Figure 1. A model of a fission reaction of uranium-235.[1] Notation that this is just one of the many possible fission reactions.
Nuclear fission is the process of splitting autonomously nuclei (normally big nuclei). When large nuclei, such as uranium-235, fissions, energy is released.[2] So much energy is released that there is a measurable decrease in mass, from the mass-energy equivalence. This means that some of the mass is converted to energy. The amount of mass lost in the fission procedure is equal to almost 3.20×10−11 J of energy. This fission process mostly occurs when a large nucleus that is relatively unstable (significant that there is some level of imbalance in the nucleus between the Coulomb force and the strong nuclear force) is struck by a low free energy thermal neutron. In improver to smaller nuclei being created when fission occurs, fission also releases neutrons.
Enrico Fermi originally dissever the uranium nuclei in 1934. He believed that sure elements could be produced by bombarding uranium with neutrons. Although he expected the new nuclei to accept larger atomic numbers than the original uranium, he institute that the formed nuclei were radioisotopes of lighter elements.[three] These results were correctly interpreted by Lise Meitner and Otto Frisch over Christmas holiday. To read this mannerly story nearly the history of nuclear science please run into this article.
Where Does the Energy Come From?
The enormous free energy that's released from this splitting comes from how hard the protons are repelling each other with the Coulomb force, barely held together by the strong force. Each proton is pushing every other proton with near 20 N of force, about the force of a hand resting on a person's lap. This is an incredibly huge force for such small particles. This huge forcefulness over a small-scale distance leads to a fair amount of released energy which is large enough to cause a measurable reduction in mass. This ways that the total mass of each of the fission fragments is less than the mass of the starting nucleus. This missing mass is known as the mass defect.[4]
Information technology is convenient to talk about the amount of energy that binds the nuclei together. All nuclei having this binding energy except hydrogen (which has just 1 proton and no neutrons). Information technology's helpful to call up about the binding energy available to each nucleon and this is called the bounden energy per nucleon. This is substantially how much energy is required per nucleon to carve up a nucleus. The products of fission are more stable, significant that it is more difficult to split them apart. Since the binding energy per nucleon for fission products is college, their full nucleonic mass is lower. The result of this college bounden energy and lower mass results in the production of free energy.[4] Essentially, mass defect and nuclear binding energy are interchangeable terms.
Use in Energy Generation
Fission of heavier elements is an exothermic reaction. Fission tin can release upward to 200 million eV compared to called-for coal which only gives a few eV. From this number solitary it is apparent why nuclear fission is used in electricity generation. Additionally, the corporeality of free energy released is much more efficient per mass than that of coal.[3] The master reason that nuclear fission is used for electricity generation is because with proper moderation and the use of command rods, the ejected costless neutrons from the fission reaction can then keep to react further with the fuel again. This then creates a sustained nuclear chain reaction, which releases adequately continuous amounts of free energy. Ane downside to the apply of fission as a method of generating electricity is the resulting daughter nuclei are radioactive. Beneath is a simulation showing how neutrons in a reactor result in fission events inside a fuel bundle. On the simulation, a red flash inside the fuel rod means a fission result occurred, while a blue wink indicates neutron absorption.
When nuclear fission is used to generate electricity, it is referred to as nuclear power. In this case, uranium-235 is used as the nuclear fuel and its fission is triggered past the absorption of a slow moving thermal neutron. Other isotopes that can be induced to fission like this are plutonium-239, uranium-233, and thorium-232.[two] For elements lighter than atomic number 26 on the periodic table nuclear fusion instead of nuclear fission yields energy. However, currently in that location is not a method that allows usa to admission the ability that fusion could produce.
References
- ↑ Wikimedia Eatables. (July 9, 2015). Nuclear Fission [Online]. Bachelor: https://upload.wikimedia.org/wikipedia/eatables/thumb/1/15/Nuclear_fission.svg/309px-Nuclear_fission.svg.png
- ↑ two.0 2.1 HyperPhysics. (July 23, 2015). Nuclear Fission [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/nucene/fission.html
- ↑ 3.0 three.1 UC Davis Chem Wiki. (July 23, 2015). Fission and Fusion [Online]. Available: http://chemwiki.ucdavis.edu/Physical_Chemistry/Nuclear_Chemistry/Fission_and_Fusion
- ↑ iv.0 4.one IEER. (July 23, 2015). Binding Energy [Online]. Bachelor: http://ieer.org/resource/factsheets/nuts-nuclear-physics-fission/
What Are Two Examples Of Unstable Atoms That Can Be Used For Nuclear Fission,
Source: https://energyeducation.ca/encyclopedia/Nuclear_fission
Posted by: folsehishey.blogspot.com


0 Response to "What Are Two Examples Of Unstable Atoms That Can Be Used For Nuclear Fission"
Post a Comment